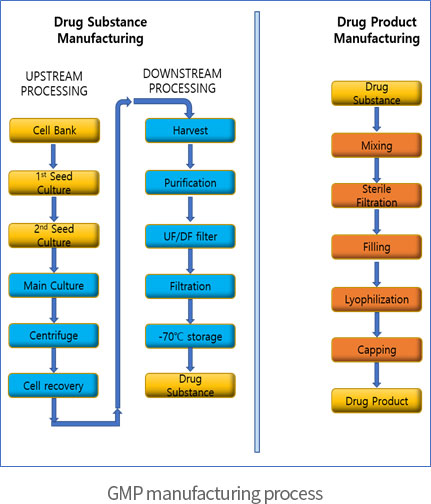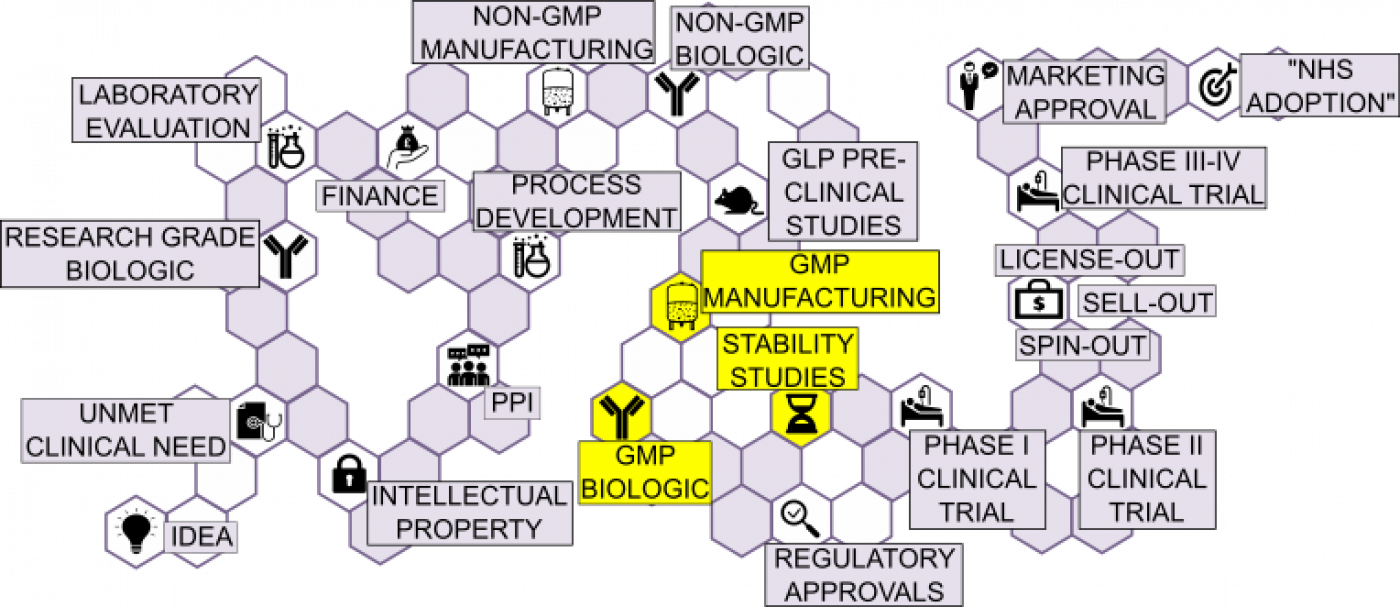
Manufacturing a Clinical Trial Product (Biologics) | UCL Therapeutic Innovation Networks - UCL – University College London

Challenges and advantages of cell therapy manufacturing under Good Manufacturing Practices within the hospital setting - ScienceDirect