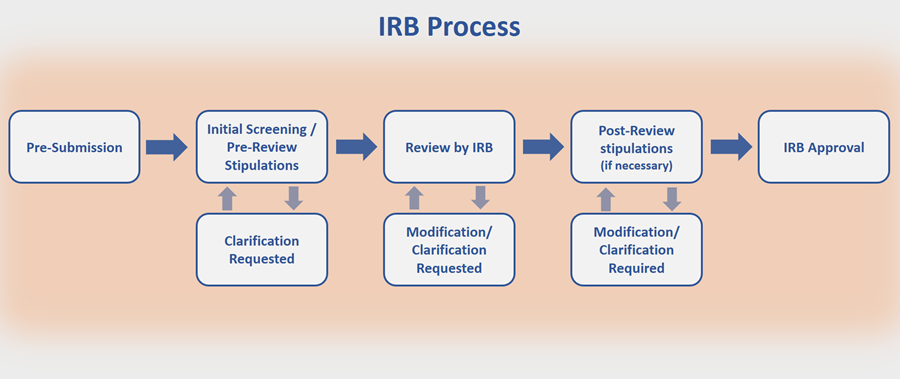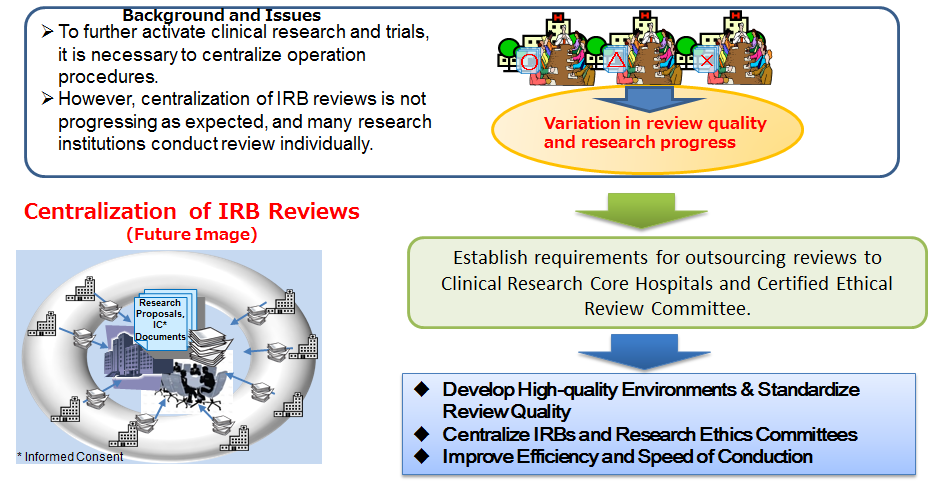
Project for Development of Central Institutional Review Board | Japan Agency for Medical Research and Development

INSTITUTIONAL REVIEW BOARD : A REFERENCE GUIDE: 9781686777486: Medicine & Health Science Books @ Amazon.com
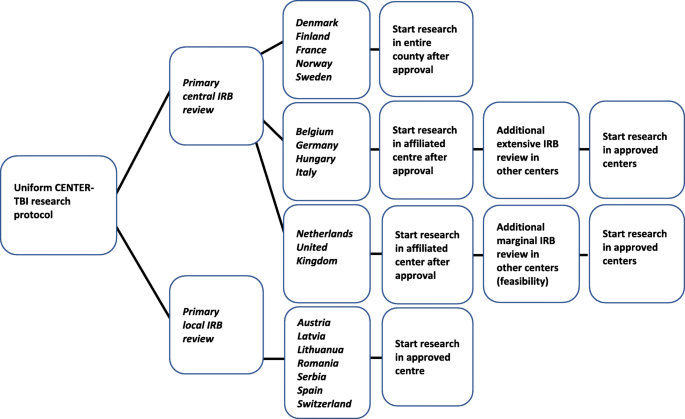
How do 66 European institutional review boards approve one protocol for an international prospective observational study on traumatic brain injury? Experiences from the CENTER-TBI study | BMC Medical Ethics | Full Text

Main Line Health - Every clinical trial conducted in the US is approved and monitored by an independent Institutional Review Board (IRB) to make sure the risks are as low as possible.