page 1 of 3 Version 1.0, 26 March 2020 Supplementary recommendations to the document European Guidance on the Management of Cl
Commission Guideline — Guidance on posting and publication of result-related information on clinical trials in relation to the
AIFA notice (update of the AIFA notice published on 12 March 2020) Clinical trials' management in Italy during the COVID-19 (

PPT - REVISION OF EUDRALEX VOL. 4 - GMP Luisa Stoppa, Ph.D. Inspection and Certification Department PowerPoint Presentation - ID:6720277

The Tao of Excellence on Twitter: "... good manufacturing practices (#GMPs) in the #clinicaltrial application, describes the content of the protocol synopsis,...3/5 #regulatoryaffairs #clinicalinvestigations #MDR #EU #Europe #clinicaltrials ...

PDF) Untangling the web of European regulations for the preparation of unlicensed radiopharmaceuticals | Clemens Decristoforo - Academia.edu

PDF) Clinical trial disclosure and transparency: Regulation EU No. 536/2014 Public disclosure at the clinical trial level
GUIDANCE DOCUMENTS CONTAINING THE COMMON PROVISIONS ON THE CONDUCT OF GCP INSPECTIONS BY COMPETENT AUTHORITIES OF THE DIFFERENT
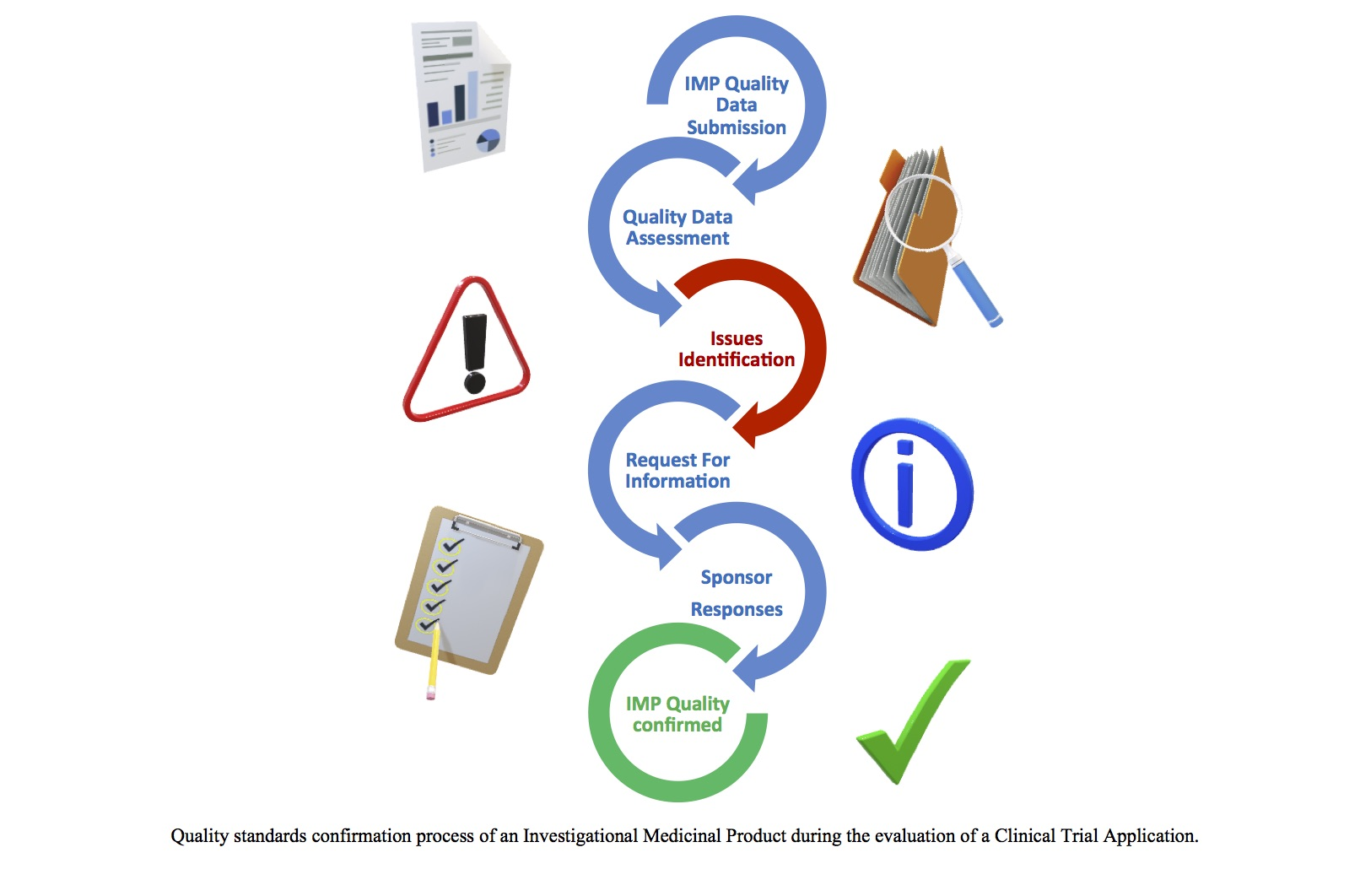
Pharmaceuticals | Free Full-Text | Quality Assessment of Investigational Medicinal Products in COVID-19 Clinical Trials: One Year of Activity at the Clinical Trials Office | HTML