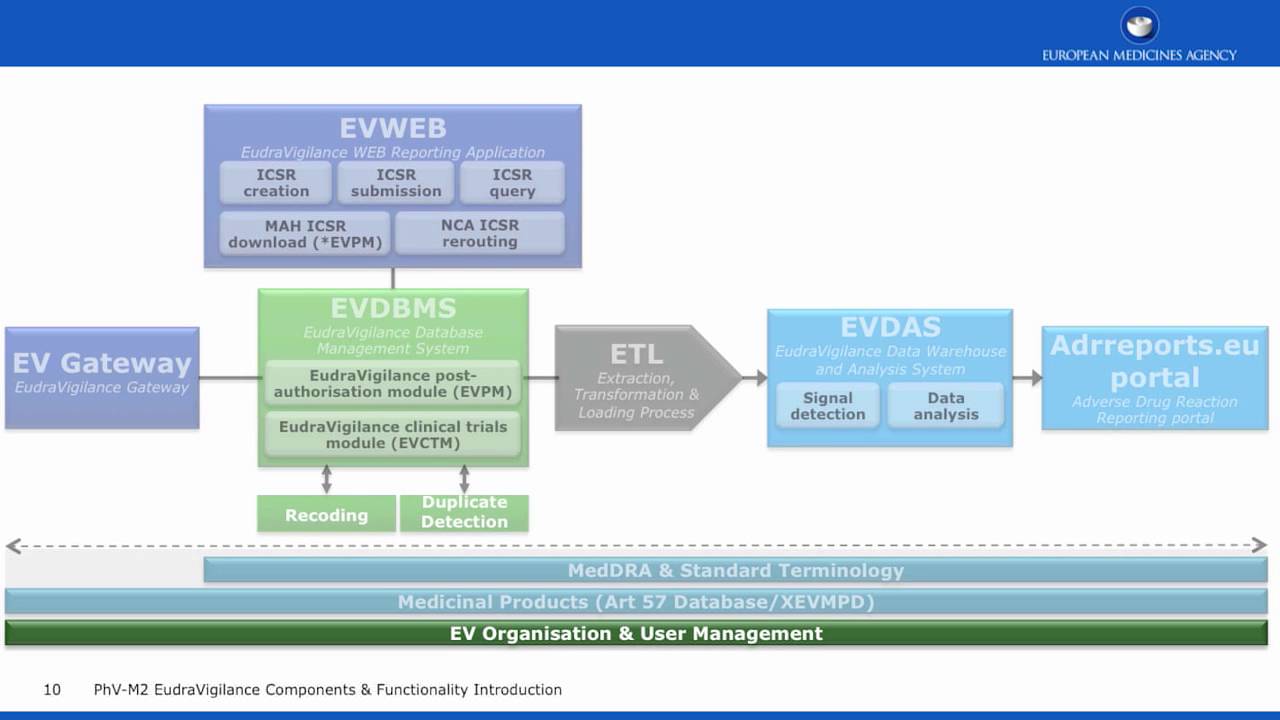
London, 01 February 2008 Doc. Ref. EMEA/553390/2007 NOTE FOR GUIDANCE EUDRAVIGILANCE HUMAN VERSION 7.1 PROCESSING OF SAFETY MESS

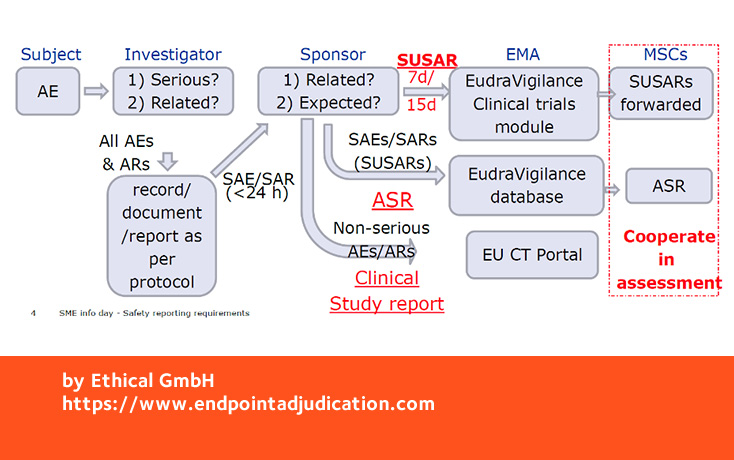
Adverse event (AE) reporting algorithm. Timeframe for adverse event... | Download Scientific Diagram

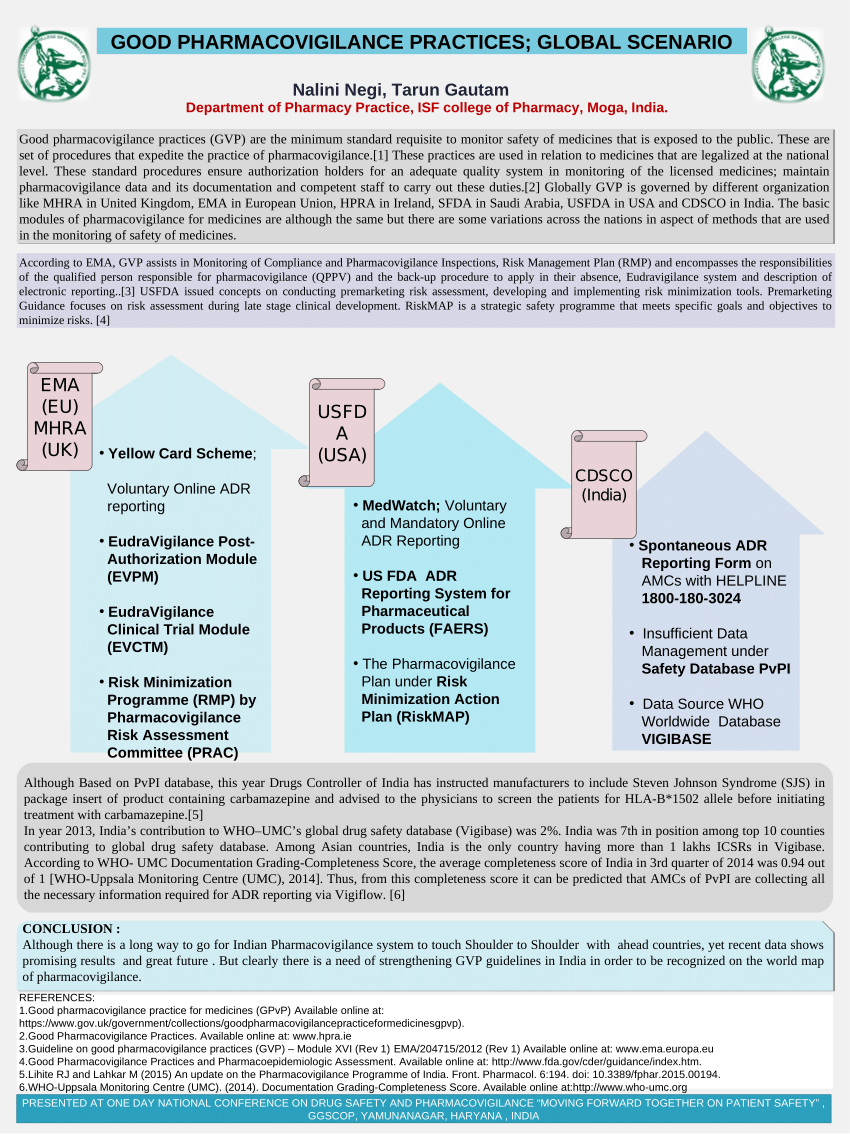
Launch of the new EudraVigilance System – National arrangements for Ireland and what this means for you | Ivowen Regulatory Affairs Specialists